Mystery Science respects the intellectual property rights of the owners of visual assets.
We make every effort to use images and videos under appropriate licenses from the owner or by
reaching out to the owner to get explicit permission. If you are the owner of a visual and
believe we are using it without permission, please
contact us—we will reply promptly and make
things right.
Exploration
lumberyard by
Image used under license from Shutterstock.com: pinyo bonmark
bench by
Image used under license from Shutterstock.com: Iablonskyi Mykola
plasticware by
Image used under license from Shutterstock.com: Kameel4u
tires by
Image used under license from Shutterstock.com: Bedrin
wrench by
Image used under license from Shutterstock.com: Tischenko Irina
glass of water by
Image used under license from Shutterstock.com: Roman Motizov
cinder blocks by
Image used under license from Shutterstock.com: Jiang Zhongyan
trees by
Image used under license from Shutterstock.com: Gerald Bernard
not allowed sign by
Image used under license from Shutterstock.com: Dmitry Natashin
window by
Image used under license from Shutterstock.com: washington1775
nitrate salts by
NurdRage
copper sulfate by
Benjah-bmm27
lead nitrate by
Ondřej Mangl
feric nitrate by
Alecjw
copper flame test by
Phillip Evans
purple fire by
Anne Helmenstine
lithium flame test by
wwwperiodictableru
different flame tests by
sciyeung
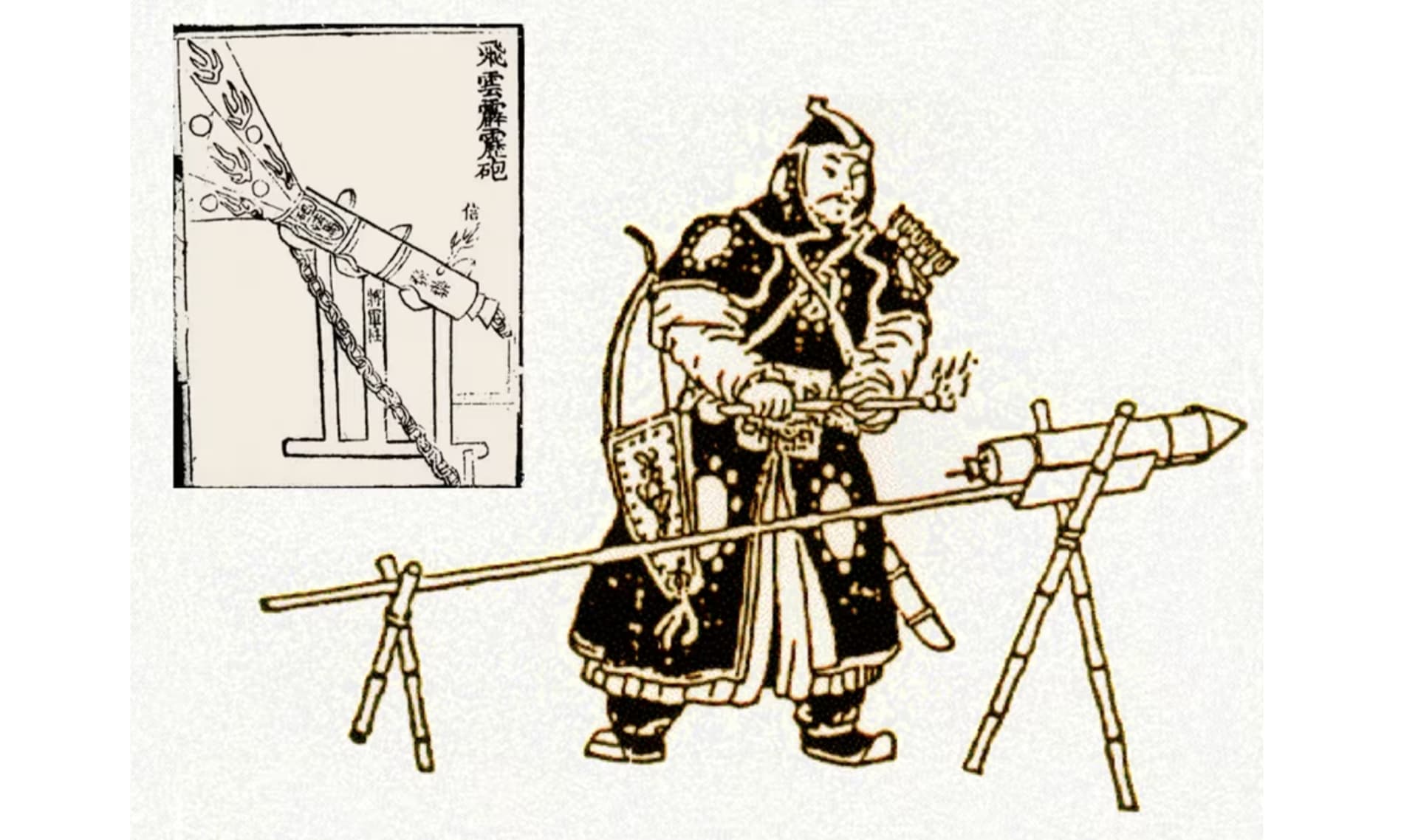
Chinese rocket by
NASA
gunpowder by
Jiao Yu and Liu Ji
Rhazes, Persian Physicist and Alchemist by
Wellcome Library
rain boots by
Image used under license from Shutterstock.com: Nataliia K
soap by
Image used under license from Shutterstock.com: Michael Kraus
stack of paper by
Image used under license from Shutterstock.com: Nuttapong
truck wheel by
Image used under license from Shutterstock.com: pema
surgeon by
Image used under license from Shutterstock.com: ChaNaWiT
rubber extraction by
gopismc
latex rubber balls by
FlinnScientific
scientist mixing chemicals by
Image used under license from Shutterstock.com: Creativa Images
Activity
play-doh by
Chrissy Southern
silly putty by
Rev. Jay Goldstein
flarp noise by
LuckyPennyShop.com
chemists by
Image used under license from Shutterstock.com: Everett Collection
scientist mixing liquid by
Image used under license from Shutterstock.com: wavebreakmedia



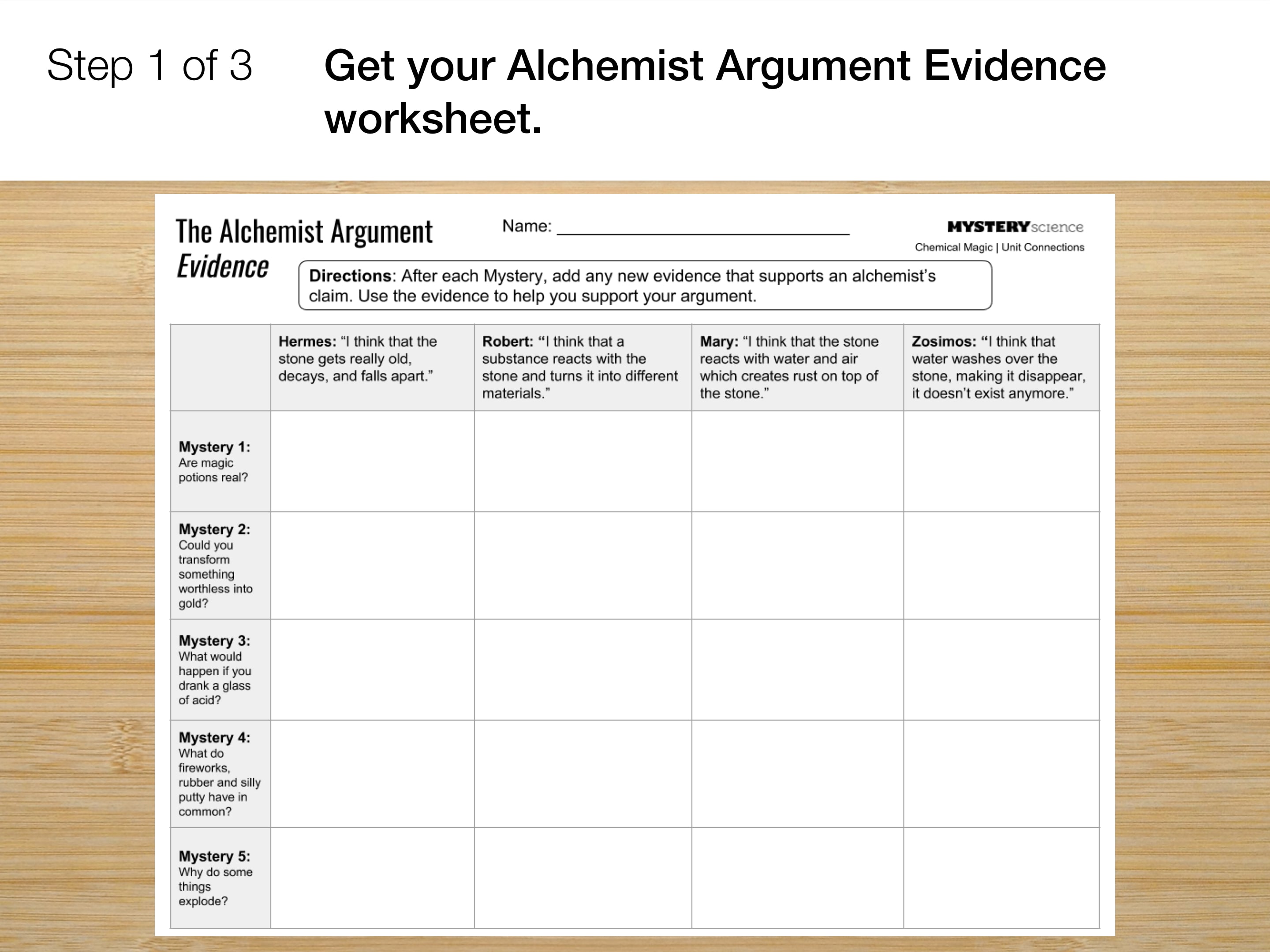
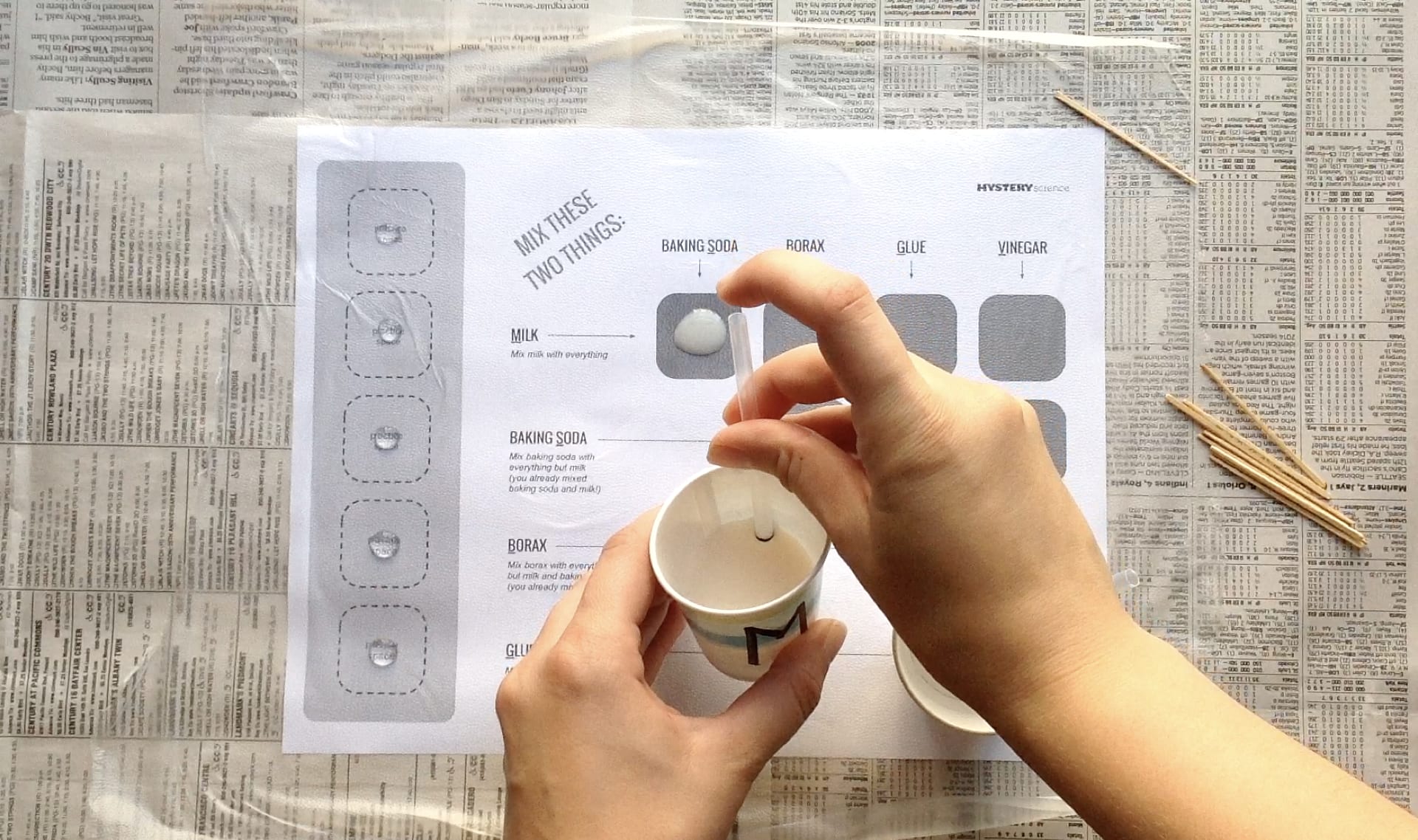
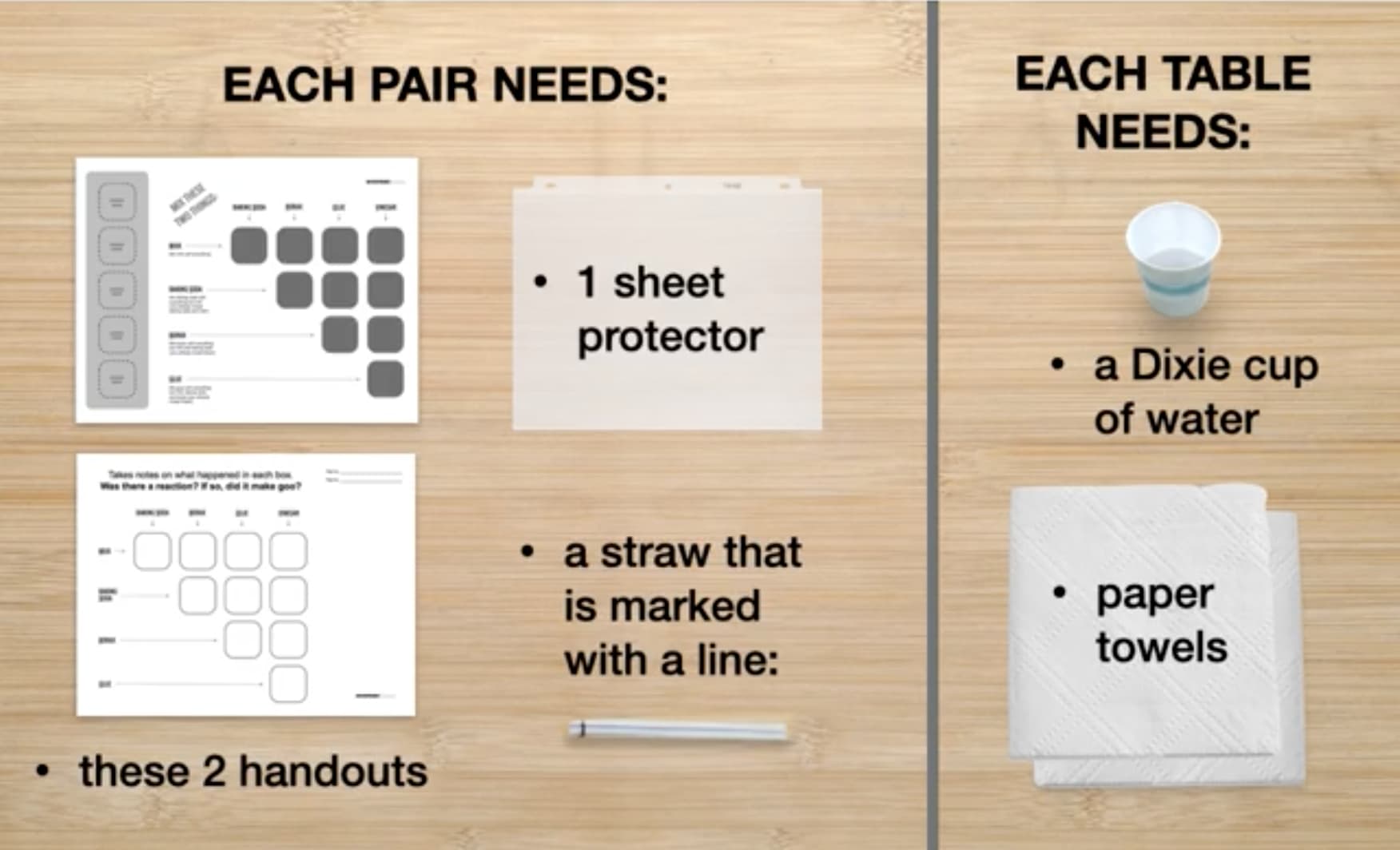
 Go to the next slide and fill in your evidence chart.
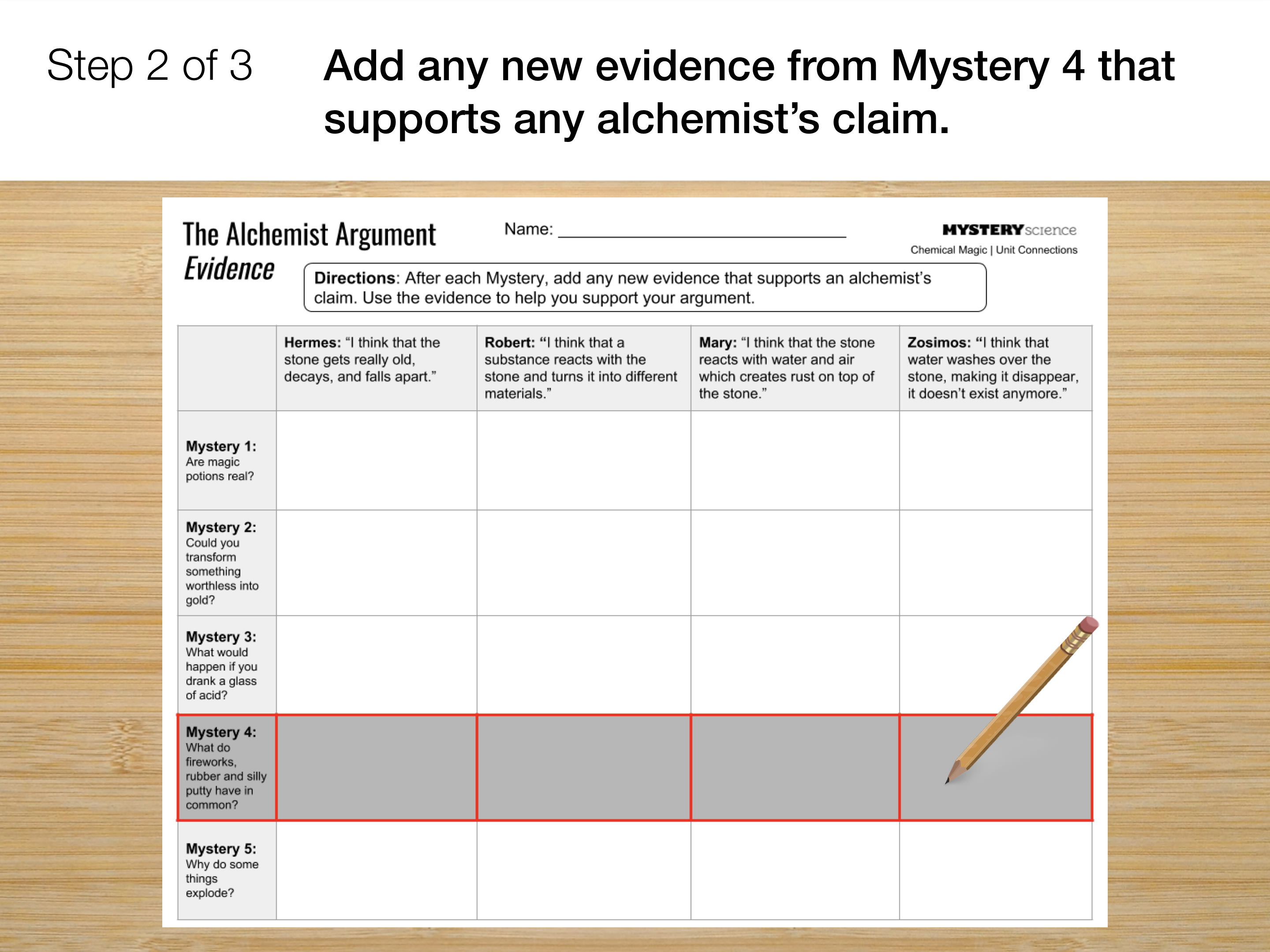
Go to the next slide and fill in your evidence chart.